Anticuerpos contra Actinobacillus pleuropneumoniae, Mycoplasma hyopneumoniae y virus de la gripe y su relación con los factores de riesgo, los signos clínicos y las lesiones pulmonares en explotaciones porcinas con sistemas de producción de un solo emplazamiento/sitio en Brasil
Abstract
A study was conducted on 21 pig herds using one-site production system in the southeast region of Brazil to assess the relationships among serological results for primary pathogens involved in respiratory diseases (Actinobacillus pleuropneumoniae, App; Mycoplasma hyopneumoniae, Mhyo; and swine influenza virus, SIV), cough index, pneumonia index, pleuritis and herd characteristics. The prevalence of antibodies against Mhyo and SIV increased throughout the raising phases, with the highest prevalence in slaughtered pigs (> 40%), while pigs in 65% (14/21) of nurseries demonstrated marked seroprevalence of App that decreased until the day of slaughter. Pleuritis and pulmonary consolidations were recorded in 9.0 and 72.4%, respectively, of the 908 evaluated lungs. Histopathological analysis of the lung lesions revealed suppurative bronchopneumonia in almost half of the lungs (48.9%). Regression analyses were conducted to identify risk factors associated with the cough index; pleuritis; pulmonary consolidation; and App, Mhyo and SIV serological results. All-in-all-out management in nursery buildings reduced the seroprevalence of Mhyo in herds. App seroprevalence was associated with pleuritis, and the presence of cough episodes in growing pigs was associated with SIV seropositivity in nursery pigs.
1. Introduction
Porcine respiratory diseases have multifactorial causes resulting from interactions among diverse etiological agents, environmental conditions and management practices and are therefore together re- ferred to as the porcine respiratory disease complex (PRDC), which remains a health challenge in the pig industry worldwide (Merialdi et al., 2012). Mycoplasma hyopneumoniae (Mhyo) and Actinobacillus pleuropneumoniae (App) are the agents most frequently related to the occurrence of suppurative bronchopneumonia and pleuritic lesions, respectively; however, other infections, such as porcine reproductive and respiratory syndrome (PRRS), swine influenza virus (SIV) infection, Pasteurella multocida infection, Haemophilus parasuis infection, and porcine circovirus type 2 (PCV-2) infection, may influence the severity of respiratory diseases (VanAlstine, 2012).
In this context, enzootic pneumonia caused by Mhyo plays a central role, and the clinical sign of dry cough in growing and finishing pigs contributes to diagnosis (Nathues et al., 2012). Coinfection with SIV may exacerbate clinical signs and promote the development of severe lesions, even in cases of viral subtypes with low pathogenicity (Thacker et al., 2001; Yazawa et al., 2004).
Infections caused by pathogenic strains of App are the primary causes of pleuritis in swine, although other agents may be involved. Pleuritic lesions and pneumonic lesions are mostly considered to occur in the cranioventral lung areas when associated with Mhyo (Rautiainen et al., 2000; Andreasen et al., 2001), while lesions in the dorsocaudal area may indicate recovery from pleuropneumonia caused by App (Jirawattanapong et al., 2010). Catarrhal bronchopneumonia affecting the cranioventral lobes and pleuritis are the most frequent macroscopic findings at the time of slaughter (Cleveland-Nielsen et al., 2002; Fablet et al., 2012; Michiels et al., 2017; Alawneh et al., 2018).
In Brazil, wide variations exist in serological data for App. In the southern region, 93% of herds have been found to be seropositive (Kich and Pontes, 2001). In another study in the southern, southeastern and west-central regions, the overall prevalence was 42.9% (1204/2808) (Moreno et al., 1999). At the time of slaughter, more than half of pigs have been found to be seropositive for Mhyo (Vicente et al., 2013; Kich and Pontes, 2001). In Brazil, SIV is the most important viral agent of PRDC, followed by PCV-2, while PRRS is a primary agent in other countries (Ciacci-Zanella et al., 2004, 2015; Rech et al., 2018); fur- thermore, studies show a wide circulation of SIV in the country, as more than 60% of farms test positive with seroprevalence greater than 70% at the herd level (Ciacci-Zanella et al., 2015).
One-site and multiple-site production systems are production strategies applied to commercial pig production. In the one-site “farrow-to- finish” system, pigs are bred and raised at the same site until slaughter, while in two-site and three-site (multiple-site) systems, pigs are raised on different farms during different rearing phases. The majority of pig farms in São Paulo State are one-site production systems run by in- dependent farmers (ABCS, 2016).
The aims of this study were to investigate the seroprevalence of App, Mhyo and SIV in nursery, growing, finishing and slaughtered pigs and to determine the prevalence of lung and pleural lesions at slaughter. Additionally, we aimed to identify the frequency of coughing in growing and finishing pigs and the risk factors related to all of them in pigs from one-site production system farms.
2. Materials and methods
2.1. Herd selection and data collection
This cross-sectional study was conducted between May 2016 and May 2017. The studied herds were located in the state of São Paulo (southeast region of Brazil), characterized by 75 pig farms using one- site rearing production system. The selection of the herds focused on farmers participating in an association of pig producers of the state (APCS, 2019). The number of farms accessed by the authors, provided by APCS, were 25 (Pinheiro-Santos, 2019), of which 21 accepted to participate in the study.
Pigs at four different phases were analyzed in each herd for the serological prevalence of App, Mhyo and SIV: the nursery phase (50 days old), the growing phase (90 days old), the finishing phase (130 days old), and at slaughter (at the age practiced by the farms; 150–180 days old, between 100 and 120 kg).
To evaluate the gross lung lesions at the time of slaughter, one trained veterinarian performed lung and pleural examinations to avoid individual variations. Each lobe was scored from 0 to 4 for pulmonary consolidations according to the method described by Piffer and Britto (1991), and the average pulmonary consolidation score was calculated per herd. Pleural lesions were classified as present or absent.
2.2. Serology
A total of 2276 serum samples were collected at the nursery (595), from growing (570) and finishing (281) pigs and at slaughter (830). The number of pigs bled in each age in the studied farms was calculated according to Noordhuizen et al. (2001); and at slaughter time, on Davies et al. (1995).
And the estimated prevalence was based Kich and Pontes (2001), a similar epidemiological survey study that analyzed herds on pig farms in southern Brazil, which results indicated 11% of seropositive samples on the nursery and growing phases and 34% on fattening pigs
The presence of antibodies against App, Mhyo and SIV was tested using the IDEXX APP-ApxIV Ab Test (99.6% specificity, 82.4% sensi- tivity), with differentiation between infected and vaccinated animals; the IDEXX M. hyo Ab Test (99.6% specificity, 89.4% sensitivity), which can detect newly acquired infections but cannot distinguish between vaccinated and infected animals; and the IDEXX Influenza A Ab Test (99.6% specificity, 95.3% sensitivity) for serotypes H1N1, H2N1, and H3N2.
Results classified as suspect in ELISAs were considered negative, since retests were not performed.
2.3. Cough examination
The cough index was determined in growing and finishing pigs. The groups of pigs were clinically examined for coughing frequency, and the index was weighted according to the maximum possible number of pigs evaluated, being at least 50% of pens evaluated in each unit in the farm. The test was performed according to the Soncini and Madureira method (1998).
A coughing episode was defined as either a single cough or a set of continuous coughs by a single animal. The coughs were counted for one minute, and the counting process was repeated three times with a one- minute interval in between. The pigs from each pen were forced to move by shouting and clapping. The average cough index (percentage) was calculated as the mean of three observations divided by the number of pigs per analyzed pen, and herds for which at least 10% of pigs were affected were considered herds with considerable respiratory problems (Soncini and Madureira, 1998).
2.4. Pneumonia scoring and histological analysis
A total of 908 lungs from slaughtered pigs of 21 herds were ana- lyzed for lung lesions. At least, 30 lungs of the first 30 slaughtered pigs per batch were scored, as previously recommended (Davies et al., 1995); the lung lesion score and pneumonia index for the severity of pulmonary consolidations were assessed using the method described by Piffer and Britto (1991).
The pulmonary consolidations in each lobe were scored from 0 to 4 depending on the percentage of the lobe affected, as follows: 0, no le- sions; 1, ≤ 25% of lobe affected; 2, between 25 and 50% of lobe af- fected; 3, between 51 and 75% of lobe affected; 4, ≥ 76% of lobe af- fected. The sum of the scores of the individual lobes was taken as the overall score, with a maximum possible score of 28.
Then, the percentage of pulmonary consolidation volume per lung was calculated and classified on seven categories: 0, no pulmonary consolidation; 1, 0.1–11% of pulmonary consolidation volume; 2, 11.1–21%; 3, 21.1–31%; 4, 31.1–41%; 5, 41.1–51%; 6, 51.1–100%. After that, the number of lungs of each category was multiplied by its own category number, then, added together named as total index.
The total index divided by the number of examined lungs resulted in the pneumonia index, which indicated the severity prevalence at the herd level. The pneumonia index included three groups according to the presumed current level of respiratory disease: low (< 0.55), mod- erate (0.55 to 0.90) and high (> 0.90). The lungs were also evaluated for the presence or absence of pleuritis, which was defined as fibrotic adherences between the parietal and visceral membranes of the pleural cavity (Rubies et al., 1999).
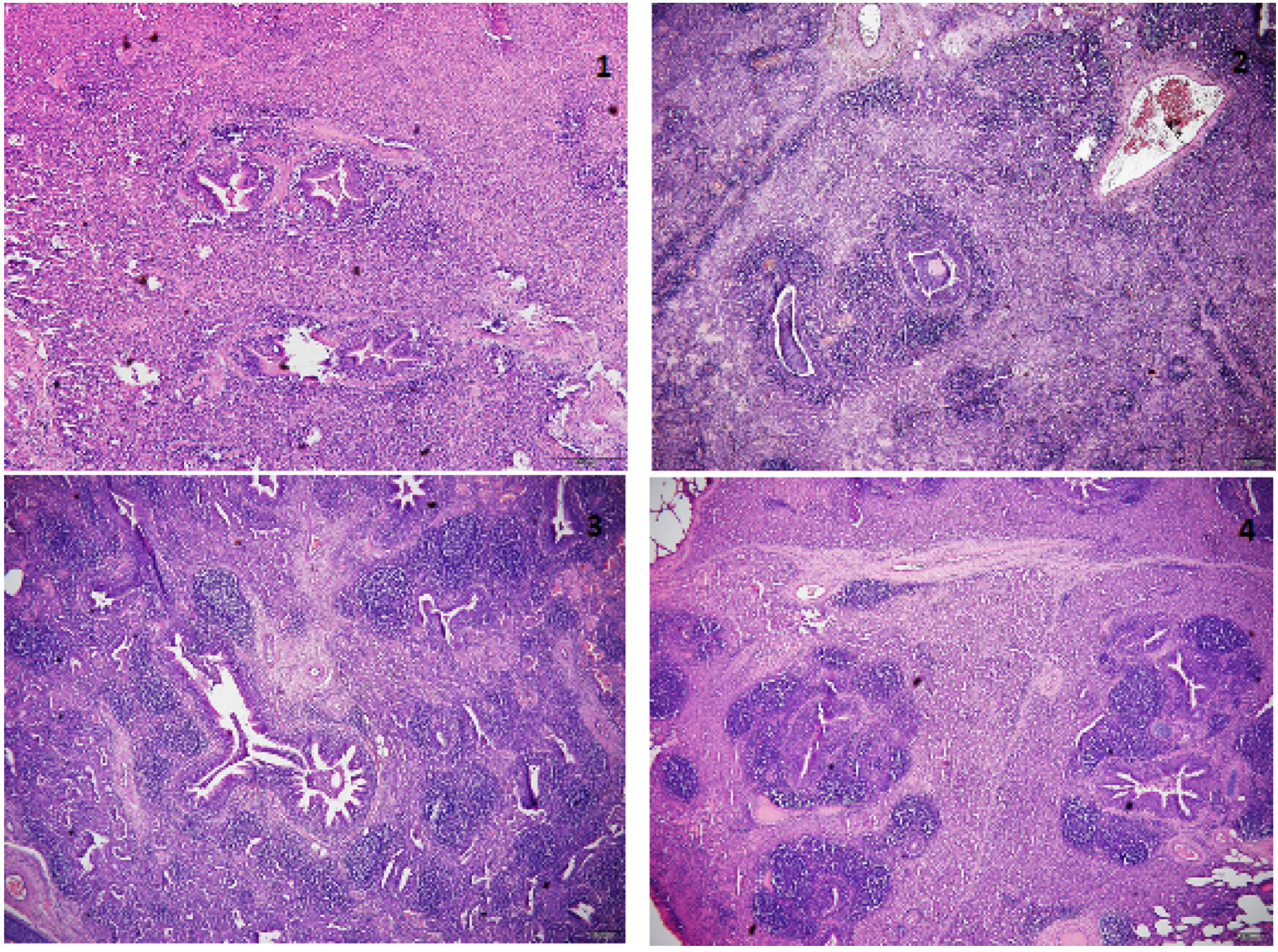
Of 908 examined lungs, 315 presented pulmonary consolidation and were collected for histopathological examination to determine the characteristics of lung lesions with special consideration of catarrhal pneumonia, alveolar interstitial pneumonia, bronchointerstitial pneu- monia, and necrotizing pneumonia. The tissues were fixed in 10% buffered formalin and embedded in paraffin, and the tissue slides were stained with hematoxylin and eosin (HE). Lymphocyte infiltration in bronchus-associated lymphoid tissue (BALT) was classified on a scale ranging from mild diffuse infiltration in peribronchial, peribronchiolar and perivascular tissues (+) to extensive presence of lymphocyte no- dules (+ + + +) (Hansen et al., 2010) (Fig. 1).
This microscopic evaluation did not reflect the prevalence of his- topathological lesions at the herd level, as lungs without macroscopic lesions were not collected and it was not possible to sample all of the lungs presenting pulmonary consolidations. The same trained veter- inarian who performed the lung score evaluation also collected the lung fragments.
2.5. Farm questionnaire
On each farm during the visit, the farmers or technical managers were questioned by the same researcher who also completed the questionnaire about farm structure, herd size, herd flow, management and biosecurity procedures, and vaccination programs; 20 farmers an- swered all questions.
For the statistical analysis, the 42 technical features from the farm questionnaire were included. All data obtained are summarized in the Supplementary Material.
2.6. Statistical analyses
To avoid considering false-positive reactions, farms with<10% positive samples were considered negative (Zimmerman et al., 1990). The serological data were used as dependent or independent variables depending on the association to be investigated.
The herd means of the variables evaluated in this study were ob- tained for values that were normally distributed, and the herd medians were obtained for values that were not normally distributed.
2.7. Associations between categorical variables
Initially, associations with P < 0.20 between categorical variables were selected using Fisher’s test. These associations were then in- vestigated using univariate logistic regression, and associations with a P < 0.05 were selected. Then, logistic regression models with multiple variables were examined. Analyses were performed using Epi InfoTM software.
The presence of pleuritis, the mean lung area affected, the pneumonia index, and the presence of cough episodes in growing and finishing pigs were the dependent variables, and the serological results for App, Mhyo and SIV were the independent variables.
In the evaluation of risk factors based on the farm questionnaire, using all-in all-out at the building level on a given farm, the presence of an external aisle in the buildings for the movements of people, and the colostrum supply to piglets were the independent variables, while the serological results for App, Mhyo and SIV were the dependent variables.
2.8. Associations between continuous variables
To investigate the existence of associations between continuous variables, simple linear regression models and multiple regression models were done using the software R. The normality of the residuals was evaluated by the Shapiro Wilk normality test.
The results of the linear regression analysis were considered valid when the required assumptions of the models, such as normality of the residuals, were met.
Analyses were conducted using the percentages of mortality in the nursery, growing and finishing periods; the number of sows per farm employee; the percentage of lungs with pleuritis at slaughter; the mean lung area affected; the pneumonia index; and the frequency of cough in growing and finishing pigs as dependent variables. The serological re- sults for App, Mhyo and SIV were used as independent variables.
The data collected on the farm questionnaires, the downtime per- iods in the maternity, nursery, growing and finishing buildings; the distance in kilometers to the nearest farm; the duration of feeding aid; and feed conversion were the dependent variables. The serological results for App, Mhyo and SIV were the independent variables.
3. Results
3.1. Serology: nursery, growing, finishing and slaughtered pigs
The herds seroconverted to App only in the nursery units, and the percentage of positive pigs decreased during the rearing period; in contrast, Mhyo and SIV seroconversion increased during rearing, reaching the highest percentages in fattening and slaughtered pigs. The App serological results showed that 3/21 herds were ser- onegative from nursery to slaughter, 17/21 herds showed decreases in the number of seropositives during the rearing phases, and only one herd presented seropositive pigs at slaughter (Table 1). In the nursery phase, 58% of pigs were seropositive for App, while 4.6% were positive at slaughter (Table 2). No antibodies against Mhyo were detected in the samples of two herds from nursery to slaughter, and 18/21 herds were positive at slaughter, presented more than half of samples positive (Table 1).
The seroprevalence of SIV gradually increased from 29.2 to 51.8% from the nursery phase to slaughter, respectively (Table 2). More than 76% positive samples were detected in the growing pigs of two studied herds, and this same seroprevalence percentage was observed in ten herds on slaughter time (Table 1).
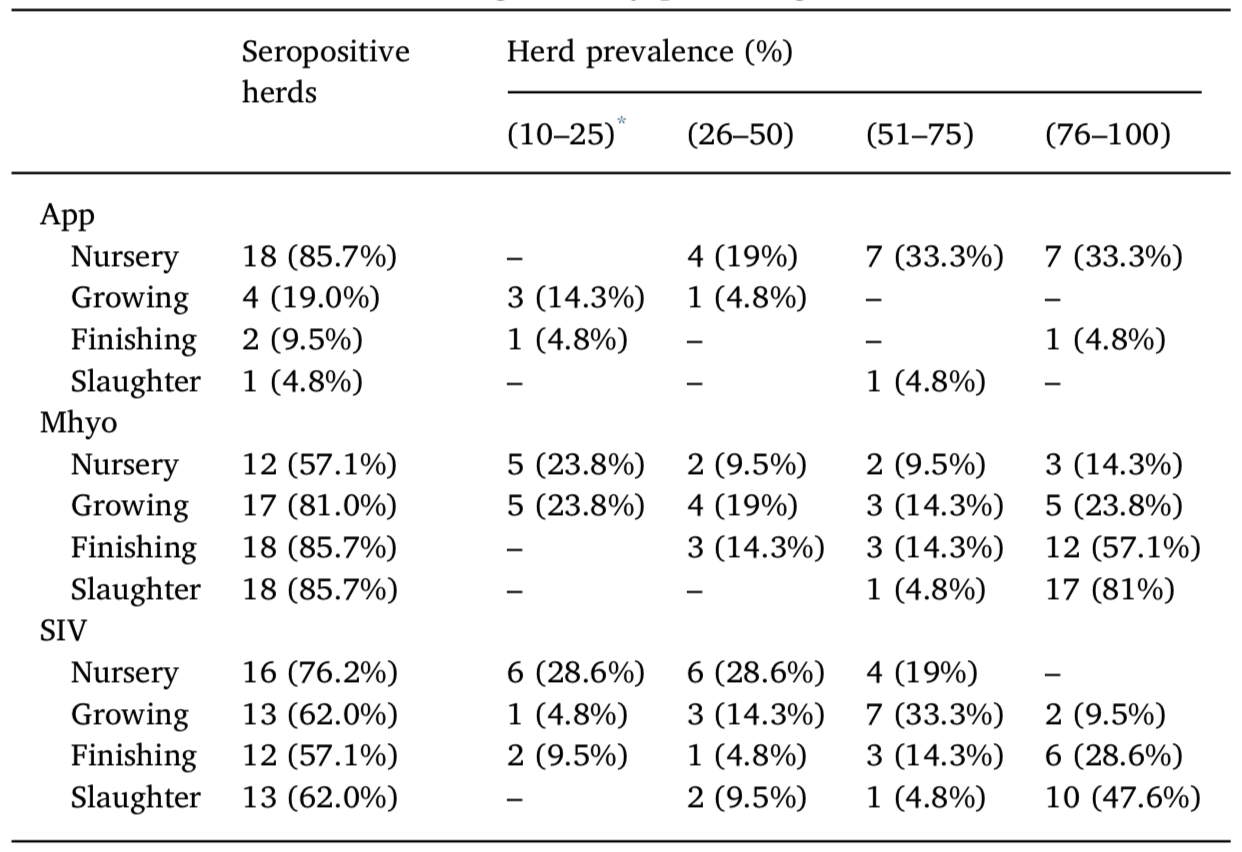
Serological results for 21 farms expressed as the total number of herds and the percentage of the total number of screened herds (21). Herd prevalence was divided into four different categories (by percentage).
App, Actinobacillus pleuropneumoniae; Mhyo, Mycoplasma hyopneumoniae; SIV, swine influenza virus.
* The herd prevalence cutoff was established as 10% because farms with < 10% seropositive samples were considered false positives (see text).
3.2. Cough index and slaughterhouse inspection
Following the cough index methodology, 4.8% (1/21) of herds at growing age and 28.5% (6/21) at finishing age presented respiratory problems (i.e., over 10% of the tested pigs were affected by cough). The mean pneumonia index was 1.47 ± 0.83. The median frequency of pleuritis was 6.7% (minimum=0, maximum=32.5%, SD=10.2), and 9.0% (82/908) of evaluated lungs presented with pleuritis at the moment of slaughter.
The results for pulmonary consolidations revealed that 72.4 ± 16.6% (657/908) of the lungs presented some degree of this type of lesion, with an average affected pulmonary area of 12%. Most of the slaughtered pig lungs, 79.8% (725/908), presented areas affected by pulmonary consolidations of less than 20%, and 27.6% (251/908) presented no lesions at slaughter age.
Histological examination of the consolidations revealed that 48.9% of lungs (154/315) demonstrated suppurative bronchopneumonia, 31.1% (98/315) demonstrated bronchointerstitial pneumonia and 81.6% (257/315) demonstrated peribronchiolar cuffing (i.e., hyper- plasia of the BALT) (Table 3).
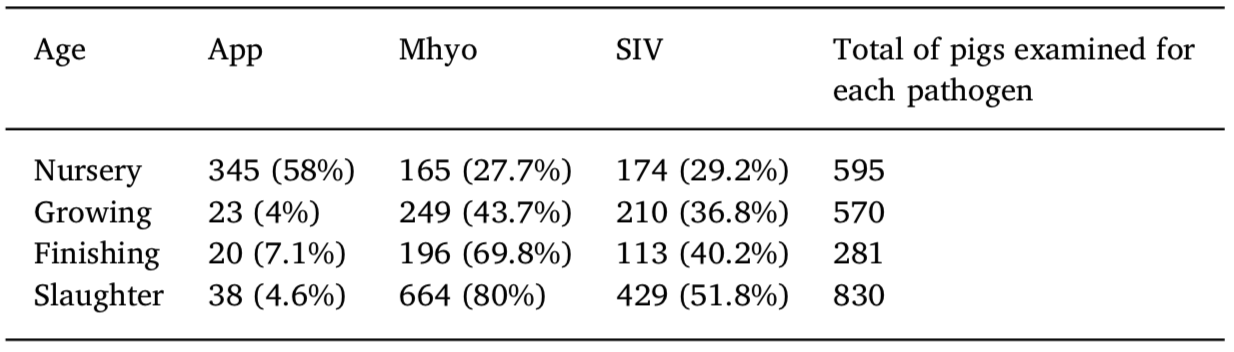
Number and percentage of positive serological results for Actinobacillus pleur- opneumoniae, Mycoplasma hyopneumoniae, and swine influenza virus as de- termined by ELISA for pigs in four distinct periods (nursery, growing, finishing, and slaughter) on 21 one-site system pig farms.
App, Actinobacillus pleuropneumoniae; Mhyo, Mycoplasma hyopneumoniae; SIV, swine influenza virus.
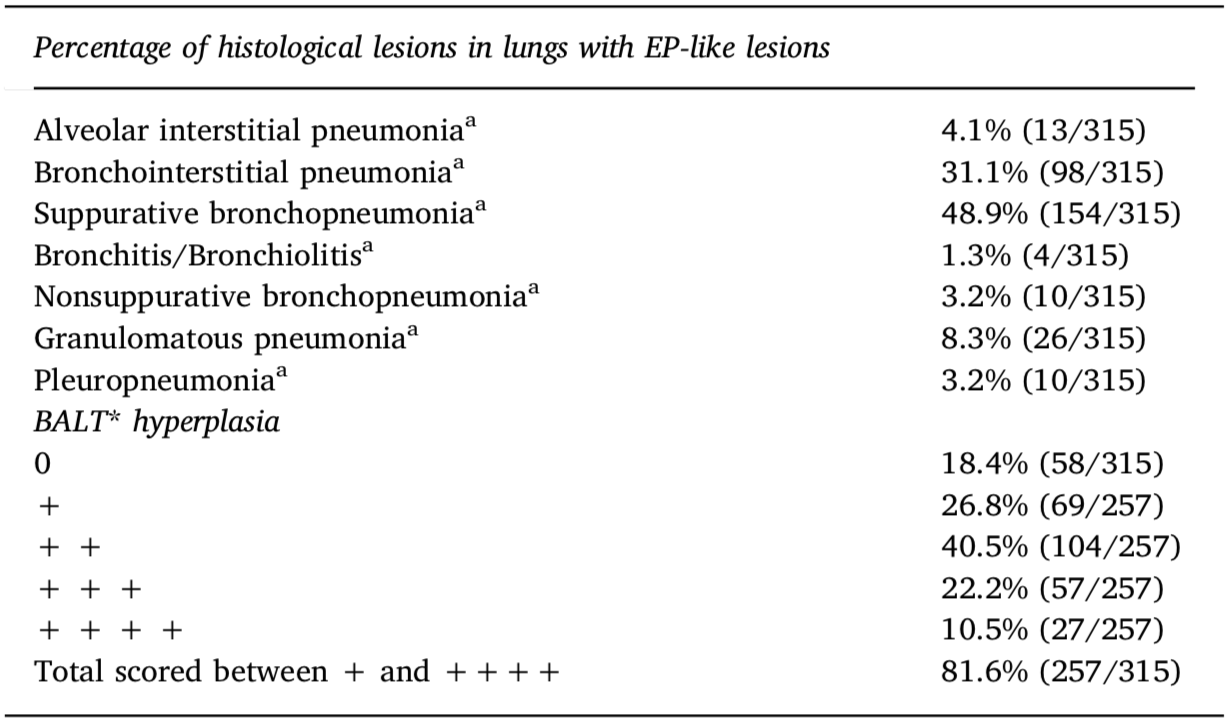
Histopathological findings in 315 lungs with enzootic pneumonia-like (EP-like) lesions from 21 studied herds at the slaughterhouse.
aSome lungs had more than one type of histopathological finding but were classified based on the most important/evident lesions.
*Lymphocyte infiltration in bronchus-associated lymphoid tissue (BALT). +BALT hyperplasia was scored as absent (0), mild (+), moderate (+ +), marked (+ + +) or extensive (+ + + +).
3.3. Farm questionnaire
Ten farms were classified as small (63–555 sows), four as inter- mediate (556–870 sows) and seven as large (≥ 871 sows).
An all-in-all-out production system was in operation in 28.6% (6/ 21) of nursery and 19% (4/21) of growing and finishing herd units, with the others, 11/21 (52.4%), applying continuous flow production. Downtime periods were utilized for 81% (17/20) of nursery and 80% (16/20) of growing/finishing pigs, with averages of 3.3 days (1–15 days) and 4.8 days (1–30 days) between batches, respectively.
Natural ventilation was used on all 21 farms. On 23.8% (5/21) of the farms, nursery and growing/finishing units had a concrete floor with no slatted area. On the farms, 76.2% (16/21) of nursery and 28.5% (6/21) of growing/finishing units had an external aisle for each com- partment. Forty percent (8/20) of the farms applied quarantine man- agement, and an average of 23 days (21–27) days was used for weaning piglets at 6.3 kg (5.5–7.1 kg).
On all farms (21/21), dry feed was supplied, including in the growing and finishing phases. Of the farms, 52.4% (11/21) recorded clinical respiratory signs in the nursery phase, and 80% (16/20) re- corded problems in pigs of growing and finishing ages.
Vaccination against Mhyo was applied in 16/20 (80%) herds, of which 62.5% (10/ 16) of herds were subjected to a one-shot protocol for 21-day-old pig- lets, while the other herds (6/16) were subjected to a two-shot protocol. In addition, 6/20 (30%) herds were subjected to App vaccination in 21- and 42-day-old piglets. Vaccination against SIV was not practiced in any of the selected herds. On 38.1% (8/21) of farms, visitors were re- quired to shower before coming into contact with the pigs. Most of the farms did not have information about App, Mhyo and SIV infection status in the pig herds before the study began, except two that stated the herds to be Mhyo free.
3.4. Logistic regression
In the analysis of qualitative variables, a significant association (P = 0.0453) and inverse correlation (odds ratio, OR = 0.0715; con- fidence interval, CI = 0.0054-0.9468) were observed between the percentages of pigs seropositive for Mhyo (dependent variable) and the use of all-in-all-out management in nursery buildings (independent variable). Additionally, a significant association (P = 0.0473) and di- rect correlation (OR = 10.4987; CI = 1.0287–107.15) were observed between the presence of cough in growing pigs (dependent variable) and the percentage of nursery pigs seropositive for SIV (independent variable).
A multivariate logistic regression model was constructed including all variables with significant associations in univariate analysis; how- ever, no significant results were obtained.
3.5. Linear regression
The seroprevalence in the herd for App (P = 0.0283) was asso- ciated with the percentage of lungs with pleuritic lesions (Y = 16.1662 + 0.6634 * App). Based on the R2 values obtained, seroconversion for these agents explained 22.9% of the pleuritic lesions observed at slaughter. The seropositivity for this same agent in nursery pigs was directly reflected by the worsening of the feed conversion rate (CA) (Y = 1.162 + 0.0173 * CA) in finishing pigs, explaining 47% of the variation observed.
A multivariate linear regression model was constructed including all variables with significant associations in univariate analysis; however, no significant results were obtained.
4. Discussion
The number of herds seropositive for App declined as pig age in- creased in the rearing system. The high seropositivity in the nursery phase might have been accentuated by colostral antibodies, which are detectable between 2 and 12 weeks of age (Gardner et al., 1991; Vigre et al., 2003), suggesting that the agent must have circulated in sow herds, as no farm vaccinated sows for App and few farms vaccinated piglets. In addition, the App ELISA had the ability to distinguish in- fected from vaccinated samples. At slaughter, only one herd was posi- tive, unlike previous studies in southern Brazil indicating a prevalence greater than 40% (Moreno et al., 1999; Kich and Pontes, 2001) and in Europe indicating a prevalence above 70% (Cleveland-Nielsen et al., 2002; Meyns et al., 2011; Fraile et al., 2010).
The high seroprevalence of Mhyo (69.8% seroprevalence in the fattening phase and 80% at slaughter) is in accordance with previous findings published by other authors in Brazil (Kich and Pontes, 2001; Vicente et al., 2013) and Europe, for which the results indicated greater than 50% seropositivity (Merialdi et al., 2012; Fablet et al., 2012; Fraile et al., 2010; Meyns et al., 2011); these findings reinforce the idea that this agent is widely disseminated in the global pig industry.
In Brazil, the vaccination against Mhyo is widespread, with few exceptions as genetic companies farm and Mhyo free herds. According to Garza-Moreno et al. (2017), vaccination against Mhyo is the most commonly used acclimation procedure in Europe, suggesting that vaccination is widely used in European swine herds except in some Mhyo- free countries, such as Switzerland.
The dynamics of Mhyo infection are not only related to the all-in all- out system but also to vaccination practices (Nathues et al., 2013) and farm type; in fact, some studies have revealed a predominant effect of farm type on Mhyo infection, with insignificant involvement of vacci- nation (Giacomini et al., 2016). On one-site farms, piglet infection tends to occur earlier, and the prevalence increases progressively with age (Maes, 2010), as observed in the present study, while on two- and three- site farms, infection occurs later but spreads faster; additionally, farm size does not influence infection dynamics (Giacomini et al., 2016).
SIV infection had a high prevalence in our study and was noted in more than half (13/21) of the sampled herds, developing from 29.2% in nursery pigs to 51.8% at slaughter. A high prevalence (≥ 50%) of SIV as an enzootic pathogen has also been reported in Europe (Van Heeth et al., 2008; Fraile et al., 2010, Fablet et al., 2012). In Brazil, studies have shown widespread circulation of this agent, with more than 70% seroprevalence and 60% positive herds (Ciacci-Zanella et al., 2015) and more than 40% seropositive sows (Rajão et al., 2013).
Pulmonary consolidations were present with high frequencies in slaughtered pigs (72.4%), consistent with the results of previous Brazilian and European studies in which similar lesions were found to have frequencies between 50 and 75% (Sobestiansky et al., 2001; Silva et al., 2001) and between 23 and 69% (Fraile et al., 2010; Meyns et al., 2011; Merialdi et al., 2012), respectively.
Notably, pulmonary consolidations are not pathognomonic of Mhyo infection since SIV and some bacterial infections also produce the same gross lesions (Palzer et al., 2008). Given that Mhyo and SIV were highly seroprevalent in the present study, particularly in the fattening and slaughter periods, it is possible to infer that these agents might be re- sponsible for this type of lung lesion in the studied slaughtered pigs.
Moreover, the histopathological analyses revealed, at the pig level, that the most prevalent lesion was suppurative bronchopneumonia, indicating the relationship between this type of lesion in slaughtered pigs and Mhyo. This result is consistent with the results of other pre- vious Brazilian and international studies demonstrating coincident in- creases in the seroprevalence of this pathogen and the incidence of this type of lung lesion (Morés et al., 2015; Hansen et al., 2010; Jirawattanapong et al., 2010).
The mean incidence of pleuritis in the herds (6.7%) in this study at slaughter was similar to that observed previously in Brazil (Silva et al., 2001) but lower than that reported in Europe, where the incidence of pleuritis was reported to be above 20% in evaluated herds (Cleveland- Nielsen et al., 2002; Meyns et al., 2011; Fraile et al., 2010).
The lack of significance on multivariate analysis, which was at- tempted in this study, regarded to the small number of studied farms; however, even with this limitation, it was possible to infer associations on univariate analysis, such as a positive association was detected be- tween the presence of cough in growing pigs and increased SIV ser- oprevalence in pigs at nursery age. Fablet et al. (2012) found that, in addition to Mhyo, SIV, PRRS, and PCV-2 were also associated with the presence of cough. Mhyo infection was not associated with the presence of cough in the present study, as expected. In previous studies, the presence of cough in pigs between 16 and 22 weeks of age was posi- tively associated with Mhyo infection and lung lesions (Morés et al., 1999; Fablet et al., 2012; Luehrs et al., 2017).
App seroprevalence was positively associated with the frequency of pleuritis at slaughter, as reported by other authors (Enoe et al., 2002, Fablet et al., 2012, Merialdi et al., 2012). Only 30% (6/20) of the herds were vaccinated against App, and vaccination was not found to be as- sociated with protection against pleuritis at slaughter.
Based on the characteristics of the farms registered on the ques- tionnaires, there was an association between the prevalence of Mhyo in growing pigs and the use of all-in-all-out management in nursery buildings. This finding is consistent with the findings of previous stu- dies demonstrating that the dynamics of this pathogen are related to this management practice (Nathues et al., 2013; Giacomini et al., 2011), and the use of this management practice is probably the most important factor in the control of enzootic pneumonia since it can in- terrupt the cycle of pathogen transmission from older to younger pigs (Clark et al., 1991).
The farms that established a vaccination protocol against Mhyo (17/ 21) did not present any fewer infected pigs during the different phases, and vaccination was not positively associated with reductions in this agent in the herds. However, a progressively increasing percentage of seropositivity occurred during the rearing process, reaching the highest numbers in slaughtered pigs.
Genetic diversity is high among Mhyo isolates, and vaccination of piglets against Mhyo does not reduce the diversity of lineages in slaughtered pigs (Michiels et al., 2017). Vaccine protection against clinical pneumonia is incomplete because the com- mercial vaccine does not prevent Mhyo colonization and does not sig- nificantly reduce Mhyo transmission in vaccinated compared with nonvaccinated populations (Meyns et al., 2006). The findings of this study confirm the observations of another field study in which the number of seropositive vaccinated pigs gradually increased until the end of the finishing period, indicating that Mhyo may continue circu- late in vaccinated animals and cause active infection (Maes et al., 1999).
Pleuritis and pulmonary consolidations are associated with sig- nificant economic losses, primarily due to reductions in growth per- formance and feed use efficiency (Gottschalk, 2012; Rautiainen et al., 2000). In the present study, no specific economic evaluation was pos- sible to determine the economic losses associated with lung diseases. App seropositivity in nursery pigs was associated with an increase in feed conversation rate in finishing pigs (P = 0.0047), consistent with the results of a previous study in which pleuropneumonia decreased daily weight gain and feed conversion efficiency (Straw et al., 1989).
5. Conclusions
This study investigated the dynamics of App, Mhyo and SIV infec- tion in pigs during the nursery, fattening and slaughter periods on farms with one-site production systems in Brazil. The results demonstrated high seroprevalence of Mhyo and SIV in fattening and slaughtered pigs raised under one-site production systems and progressive circulation of these agents during the rearing process. The same results were not observed for App seroprevalence, which remained low, except in nursery pigs.
The seroprevalence of SIV at the nursery phase was positively as- sociated with the presence of cough in growing pigs. All-in-all-out management in nursery buildings was positively associated with re- duced Mhyo seroprevalence in growing pigs. Additionally, this study supported the associations between increasing App seroprevalence and increasing pleural lesions and between increasing App seroprevalence and worsening feed conversation rates.
Pulmonary consolidations were frequently observed in pigs at abattoirs of one-site production systems, the lungs of which presented with suppurative bronchopneumonia on histological examination.
Animal welfare
Permission to conduct the field study was obtained from the Ethics Committee on the Use of Animals (Protocol #22516/15), and consent was obtained from the owners of the herds.
Declaration of Competing Interest
Not applicable.
Acknowledgments
Ourofino Animal Health and a grant from São Paulo Research Foundation – FAPESP (#2015/25318-9) funded this study. The authors are grateful for the kindness and collaboration of the producers and thank the technical managers for their cooperation in providing the valuable data used in this study.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.prevetmed.2019. 104748.
References
ABCS, 2016. Mapping of Brazilian Pork Chain, first ed. Brasilia, Federal District. Alawneh, J.I., Parke, C.R., Lapuz, E.J., David, J.E., Basinang, V.G., Baluyut, A.S., Barnes,
T.S., Villar, E.C., Lopez, M.L., Meers, J., Blackall, P.J., 2018. Prevalence and risk Factors associated with gross pulmonary lesions in slaughtered Pigs in smallholder and commercial Farms in two provinces in the Philippines. Front. Vet. Sci. 5, 7.
Andreasen, M., Mousing, J., Thomsen, L.K., 2001. No simple association between time elapsed from seroconversion until slaughter and the extent of lung lesions in Danish swine. Prev. Vet. Med. 52, 147–161.
APCS, 2019. Association of pig producers of Sao Paulo. (accessed 03 May 2019). http:// www.apcs.com.br/portal/sobre-a-apcs.php.
Ciacci-Zanella, J.R., Schaefer, R., Gava, D., Haach, V., Cantao, M.E., Coldebella, A., 2015. Influenza a virus infection in brazilian swine herds following the introduction of pandemic 2009 H1n1. Vet. Microbiol. 180, 118–122.
Ciacci-Zanella, J.R., Trombetta, C., Vargas, I., Da Costa, D.E., 2004. Lack of evidence of porcine reproductive and respiratory syndrome virus (PRRSV) infection in domestic swine in Brazil. Ciência Rural 34 (2), 449–455.
Clark, L., Freeman, M., Scheidt, A., Knox, K., 1991. Investigating the transmission of Mycoplasma hyopneumoniae in a swine herd with enzootic pneumonia. Vet. Med. 86, 543–550.
Cleveland-Nielsen, A., Nielsen, E.O., Ersboll, A.K., 2002. Chronic pleuritis in Danish slaughter pig hers. Prev. Vet. Med. 55, 121–135.
Davies, P.R., Bahnson, P.B., Grass, J.J., Marsh, W.E., Dial, G.D., 1995. Comparison of methods for measurement of enzootic pneumonia lesions in pigs. Am. J. Vet. Res. 56, 709–714.
Enoe, C., Mousing, J., Schirmer, A.L., Willeberg, P., 2002. Infectious and rearing system related risk factors for chronic pleuritis in slaughter pigs. Prev. Vet. Med. 54, 337–349.
Marois, C., Fablet, C., Dorenlor, V., Eono, F., Eveno, E., Jolly, J.P., Le Devendec, L., Kobisch, M., Madec, F., Rose, N., 2012. Bacterial pathogens associated with lung lesions in slaughter pigs from 125 herds. Res. Vet. Sci. 93, 627–630.
Fraile, L., Alegre, A., López-Jiménez, R., Nofrarías, M., Segalés, J., 2010. Risk factors associated with pleuritis and cranio-ventral pulmonary consolidation in slaughter pigs. Vet. J. 184, 326–333.
Gardner, I.A., Boss_e, J.T., Sheldrake, R.F., Rosendal, S., Johnson, R.P., 1991. Serological response to Actinobacillus pleuropneumoniae serovar 7 infection in a commercial pig herd. Aust. Vet. J. 68, 349–352.
Garza-Moreno, L., Segalés, J., Pieters, M., Romagosa, A., Sibila, M., 2017. Survey on Mycoplasma hyopneumoniae gilt acclimation practices in Europe. Porcine Health Manag. 3 (1), 21.
Giacomini, E., Ferro, P., Nassuato, C., Salogni, C., Alborali, L., 2011. Dynamics of Mycoplasma hyopneumoniae infection in 4 Italian swine farrow-to-finish herds. Proceedings of the Società Italiana di Patologia ed Allevamento dei Suini.
Giacomini, E., Ferrari, N., Pitozzi, A., Remistani, M., Giardiello, D., Maes, D., Alborali, G.L., 2016. Dynamics of Mycoplasma hyopneumoniae seroconversion and infection in pigs in the three main production systems. Vet. Res. Commun. 40 (2), 81–88.
Gottschalk, M., 2012. Actinobacillosis. In: Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W. (Eds.), Diseases of Swine, tenth ed. The Iowa State University Press, Ames, Iowa, USA, pp. 653–669.
Hansen, M.S., Pors, S.E., Jensen, H.E., Bille-Hansen, V., Bisgaard, M., Flachs, E.M., Nielsen, O.L., 2010. An investigation of the pathology and pathogens associated with porcine respiratory disease complex in Denmark. J. Comp. Pathol. 143 (120), 131.
Jirawattanapong, P., Stockhofe-Zurwieden, N., van Leengoed, L.A.M.G., Wisselink, H.J., Raymakers, R., Cruijsen, T., van der Peet-Schwering, C., Nielen, M., van Nes, A., 2010. Pleuritis in slaughter pigs: relations between lung lesions and bacteriology in 10 herds with high pleuritis. Res. Vet. Sci. 88, 11–15.
Kich, J.D., Pontes, A.P., 2001. Análise da situação atual das doenças respiratórias no Brasil. http://ainfo.cnptia.embrapa.br/digital/bitstream/item/187649/1/ digitalizar0070.pdf.
Luehrs, A., Siegenthaler, S., Grützner, N., Grosse Beilage, E., Kuhnert, P., Nathues, H., 2017. Occurrence of Mycoplasma hyorhinis infections in fattening pigs and association with clinical signs and pathological lesions of Enzootic Pneumonia. Vet. Microbiol. 203, 1–5.
Maes, D., 2010. Mycoplasma hyopneumoniae in pigs 2010. Update on epidemiology and control. Proceedings of the International Pig Veterinary Society Congress.
Maes, D., Deluyker, H., Verdonck, M., Castryck, F., Miry, C., Vrijens, B., Verbeke, W., Viaene, J., De Kruif, A., 1999. Effect of vaccination against Mycoplasma hyopneumo- niae in pig herds with an all-in/all-out production system. Vaccine 17, 1024–1034.
Merialdi, G., Dottori, M., Bonilauri, P., Luppi, A., Gozio, S., Pozzi, P., Spaggiaria, B., Martelli, P., 2012. Survey of pleuritis and pulmonary lesions in pigs at abattoir with focus on the extent of the conditions and herd risk factors. Vet. J. 193, 234–239.
Meyns, T., Dewulf, J., De Kruif, A., Calus, D., Haesebrouck, F., Maes, D., 2006. Comparison of transmission of Mycoplasma hyopneumoniae in vaccinated and non- vaccinated populations. Vaccine n 24, 7081–7086.
Meyns, T., Van Steelant, J., Rolly, E., Dewulf, J., Heasebrouk, F., Maes, D., 2011. A cross- sectional study of risk factors associated with pulmonary lesions in pigs at slaughter. Vet. J. 187, 388–392.
Michiels, A., Vranckx, K., Piepers, S., Sacristán, R.P., Arsenakis, I., Boyen, F., Haesebrouck, F., Maes, D., 2017. Impact of diversity of Mycoplasma hyopneumoniae strains on lung lesions in slaughter pigs. Vet. Res. 48, 1.
Moreno, A.M., Barbarini Junior, O., Baccaro, M.R., 1999. Levantamento sorológico para Actinobacillus pleuropneumoniae em criações de suínos no período de dezembro de 1996 a julho de 1999. Proceedings: Brazilian Congress of Swine Specialists.
Morés, M.A., Oliveira Filho, J.X., Rebelatto, R., Klein, C.S., Barcellos, D.E., Coldebella, A.,Morés, N., 2015. Aspectos patológicos e microbiológicos das doenças respiratórias em
suínos de terminação no Brasil. Pesquisa Veterinária Brasileira 35 (8), 725–733. Morés, N., Sobestiansky, J., Dalla Costa, O.A., Barioni Jr, W., Piffer, I.A., Guzzo, R.,
Coimbra, J.B., 1999. Utilização da contagem de tosse e espirro como indicadores da ocorrência e severidade de pneumonias e rinite atrófica, respectivamente. Embrapa Suínos e Aves.
Nathues, H., Doehring, S., Woeste, H., Fahrion, A.S., Doherr, M.G., Grosse, B.E., 2013. Individual risk factors for Mycoplasma hyopneumoniae infections in suckling pigs at the age of weaning. Acta Vet. Scand. 55, 44.
Nathues, H., Woeste, H., Doehring, S., Fahrion, A., Doherr, M., Grosse Beilage, E., 2012. Detection of Mycoplasma hyopneumoniae in nasal swabs sampled from pig farmers. Vet. Rec. 170, 623.
Noordhuizen, J.P.T.M., Frankena, K., Thrusfield, M.V., Graat, E.A.M., 2001. Application of Quantitative Methods in Veterinary Epidemiology. Wageningen Pers, The Netherlands p. 50, 221–228.
Palzer, A., Ritzmann, M., Wolf, G., Heinritzi, K., 2008. Associations between pathogens in healthy pigs and pigs with pneumonia. Vet. Rec. 162, 267–271.
Piffer, I.A., Brito, J.R.F., 1991. Descrição de um modelo para avaliação e quantificação de lesões pulmonares em suínos e formulação de um índice para classificação de re- banhos. Concórdia: Embrapa Suínos e Aves 1–11.
Pinheiro-Santos, J.G., 2019. Diagnosis of Conformity to Minimum Standards of Quality and Detection of Improvement Points to Increased Productivity in Pig Farms. Thesis, Master of Veterinary Medicine. Sao Paulo State University, Pirassununga, Brazil.
Rajão, D.S., Alves, F., Del Puerto, H.L., Braz, G.F., Oliveira, F.G., Ciacci-Zanella, J.R., Schaefer, R., Reis, J.K.P., Guedes, R.M.C., Lobato, Z.I.P., Leite, R.C., 2013. Serological evidence of swine influenza in Brazil. Influenza Other Respir. Viruses 7, 109–112.
Rautiainen, W., Virtala, A., Wallgren, P., Saloniemi, H., 2000. Varying effect of infections with Mycoplasma hyopneumoniae on the weight gain recorded in three different multisource fattening pig herds. J. Vet. Med. B Infect. Dis. Vet. Public Health 47, 461–469.
Rech, R.R., Gava, D., Silva, M.C., Fernandes, L.T., Haach, V., Ciacci-Zanella, J.R., Schaefer, R., 2018. Porcine respiratory disease complex after the introduction of H1N1/2009 influenza virus in Brazil. Zoonoses Public Health 65, 155–161.
Rubies, X., Kielstein, P., Costa, L., Riera, P., Artigas, C., Espuna, E., 1999. Prevalence of Haemophilus parasuis serovars isolated in Spain from 1993 to 1997. Vet. Microbiol. 66 (3), 245–248.
Silva, A., Paganini, F., Acosta, J., Rocha, P., Mistura, H., Marcon, E., Simon, V., Casagrande, H., 2001. Estudo do perfil das doenças respiratórias nas regiões Sul, Sudeste e Centro-oeste do Brasil. Proceedings: Brazilian Congress of Swine Specialists, 2001.
Sobestiansky, J., Costa, O.A.D., Morés, N., Júnior, W.B., Piffer, I.A., Guzzo, R., 2001. Estudos ecopatológicos das doenças respiratórias dos suínos: prevalência e impacto econômico em sistemas de produção dos estados de Santa Catarina, Rio Grande do Sul e Paraná. Embrapa Suínos e Aves. Available on:http://docsagencia.cnptia. embrapa.br/suino/comtec/cot287.pdf(acessed 15 Jul. 2015).
Soncini, R.A., Madureira Júnior, S.E., 1998. Monitorias sanitárias. In: Sobestiansky, J., Wentz, I., Silveira, P.R.S., Sesti, L.A.C. (Eds.), 1998. Suinocultura intensiva: produção, manejo e saúde do rebanho. Embrapa, Brasília, pp. 91–110.
Straw, B., Tuovinen, V., Bigras-Poulin, M., 1989. Estimation of the cost of pneumonia in swine herds. Journal of the American Veterinary Association 195, 1702–1706. Thacker, E.L., Thacker, B.J., Janke, B.H., 2001. Interaction between Mycoplasma hyop-
neumoniae and swine influenza virus. J. Clin. Microbiol. 39, 2525–2530.
Van Reeth, K., Brown, I.H., Dürrwald, R., Foni, E., Labarque, G., Lenihan, P., Maldonado, J., Markowska-Daniel, I., Pensaert, M., Pospisil, Z., Koch, G., 2008. Seroprevalence of
H1N1, H3N2 and H1N2 influenza viruses in pigs in seven European countries in
2002–2003. Influenza Other Respir. Viruses 2, 99–105.
VanAlstine, W.G., 2012. Respiratory system. In: Zimmerman, J.J., Karriker, L.A., Ramirez,
A., Schwartz, K.J., Stevenson, G.W. (Eds.), Diseases of Swine, tenth ed. The Iowa State
University Press, Ames, Iowa, USA, pp. 348–362.
Vicente, A.F., Catto, D., Allendorf, S.D., Garcia, K., Antunes, J.M.A.P., Appolinario, C.,
Megid, J., 2013. Soropositividade para Mycoplasma hyopneumoniae em suínos aba- tidos em frigoríficos da região central do estado de São Paulo. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 1899–1903.
Vigre, H., Ersbøll, A.K., Sørensen, V., 2003. Decay of acquired colostral antibodies to Actinobacillus pleuropneumoniae in pigs. J. Vet. Med. B Infect. Dis. Vet. Public Health 50, 430–435.
Yazawa, S., Okada, M., Ono, M., Fujii, S., Okuda, Y., Shibata, I., Kida, H., 2004. Experimental dual infection of pigs with an H1N1 swine influenza virus (A/Sw/Hok/ 2/81) and Mycoplasma hyopneumoniae. Vet. Microbiol. 98, 221–228.
Zimmerman, W., Staeger, M., Bommeli, W., 1990. A seroepidemiological study on the occurrence of serotypes of A. pleuropneumoniae(APP) on Swiss breeding herds sur- veyed by pig health service. Proceedings 11th IPVS Congress 32.
